A young marine sediment was cored and installed in a laboratory column. Pyrite in the sediment was oxidized injecting H2O2 in MgCl2-solution. The produced sulfuric acid dissolved calcite. MgCl2 solution was used to precisely clarify the cation exchange reactions.

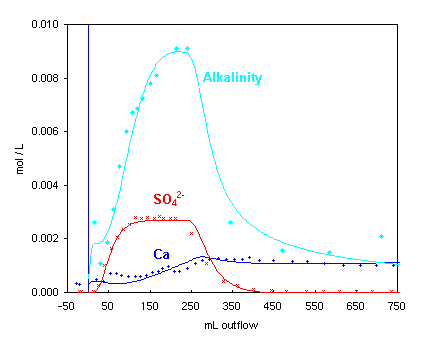
Injection of the peroxide solution starts at 0 mL.
Peroxide oxidizes pyrite:
7.5 H2O2
+ FeS2
→ Fe(OH)3 +
4 H+ +
2 SO42- +
4 H2O
After 55 mL (the column pore volume) the SO4
concentration is halfway of the maximum.
The generated acid dissolves calcite:
H+ + CaCO3
→ Ca2+ + HCO3-
As a result, the alkalinity increases. Ca2+ does not increase as much
since it exchanges with Mg:
Ca2+ + Mg-X2
↔ Ca-X2 + Mg2+
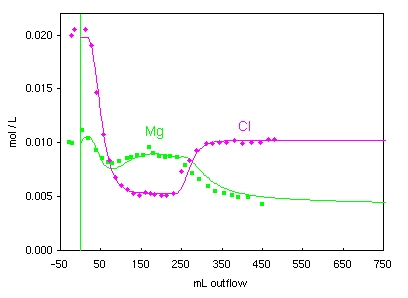
Initially, 10 mM MgCl2 solution was injected to create a uniform
exchange complex containing only Mg-X2 (concentrations before the injection of peroxide,
at negative outflow axis).
The peroxide was injected in 2.5 mM MgCl2
(note the decrease of Cl, which is a conservative tracer). After an initial decrease,
Mg increased by cation exchange.
The experiment ended with injection of 5 mM MgCl2 without peroxide.
The SO4 concentration decreases to zero, and Mg and Cl change rapidly
to the concentrations in the injected solution. Note that the Ca concentration remains steady due to ion
exchange and calcite dissolution assisted by proton exchange.
Synbols in the 2 graphs indicate measured concentrations, lines are modeled (PHREEQC file pyrite.phr).
Actually, some of PHREEQC-2 was programmed to be able to model this experiment, including:
The experiment is described in detail by Appelo, Verweij and Schäfer, 1998; 1.1 MB pdf).