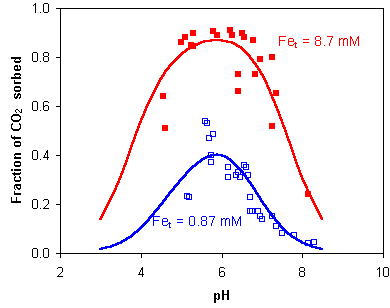
Van Geen et al. (1994, GCA 58, 2073) determined sorption of carbonate on goethite as function of pH.
They concludeds that the reactive surface sites of metal oxides in natural waters may be
predominantly covered by carbonate species. The lower availability of sorption sites affects
sorption of trace metals such as Cd, Cu, Ni and As, and thus influences the mobility of these
heavy metals in the natural environment.
The de facto standard database of Dzombak and Morel (1990) for sorption of heavy metals
on hydrous ferric oxide (HFO) does not contain data on CO2 species,
Applications of geochemical models such as MINTEQ and PHREEQC which use this database may be in
serious error when sorption of a major ion is neglected.
The carbonate sorption data obtained by Zachara et al. (1987, EST 21, 589) for ferrihydrite were
optimized to the Dzombak and Morel database by Appelo,
Van der Weiden, Tournassat and Charlet (2002); 98 kB pdf.
