We consider the column discussed in chromatographic displacement ,
with kinetic exchange among sorbed and solute ions.
Ion exchange is a fast process, the half-life lies in the order of milliseconds. Exchange can normally be calculated
as an equilibrium reaction.
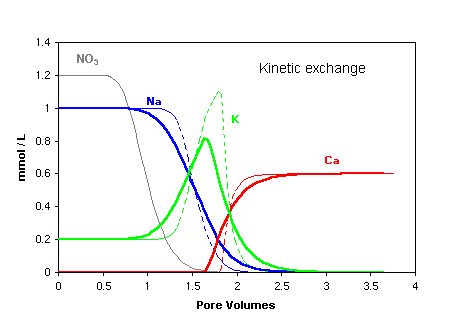
However, kinetic exchange can be important for trace metals when several sites sorb the metal with varying strength.
Kinetic effects show up in early front arrival and front tailing, similar to the effects of
stagnant zones. However, the fronts of anions and tracers like tritium
are not affected by kinetic exchange and follow the pattern of a homogeneous column.

The PHREEQC input file chro_kin.phr simulates the outflow from the column with kinetic exchange. Simple first order rates are defined according to:
RATES
kin_exch_na
# kinetic exchange: d(q_Na)/dt = k * (q_Na_eq - q_Na)
# q_Na_eq is equilibrium exchangeable Na
-start
10 q_Na_eq = 1e7 * mol("NaX")
20 q_Na = m
30 dq_Na = 100 * (q_Na_eq - q_Na) * time
40 save -dq_Na
-end
The equilibrium concentration is obtained in line 10. mol("NaX") stands for a special BASIC function of
PHREEQC that gives exchangeable Na in equilibrium with the solution.
It is calculated for a tiny concentration of exchangeable X, 10-7 smaller
than the actual concentration, defined under keyword EXCHANGE.
The small exchanger concentration has negligible influence on solute concentrations when exchanging with
the solution.
Multiplying with 107 in line 10 gives the equilibrium concentration of NaX.