Clays can act as semipermeable membranes by virtue of the diffuse double layer (DDL) in the pores. In the DDL, the negative charge of the clay minerals is compenstated by an excess of cations and a deficit of anions. At low ionic strength, the DDL may extend all through the pore, and diffusion of anions in the clay is restricted. But, diffusion of cations is then hindered as well by electroneutrality. PHREEQC can calculate the thickness of the DDL as a function of ionic strength, and the composition of the DDL by Donnan equilibrium. The multicomponent module calculates diffusion both in the free pore solution and in the DDL. Together, these options allow to simulate diffusion in clay membranes.

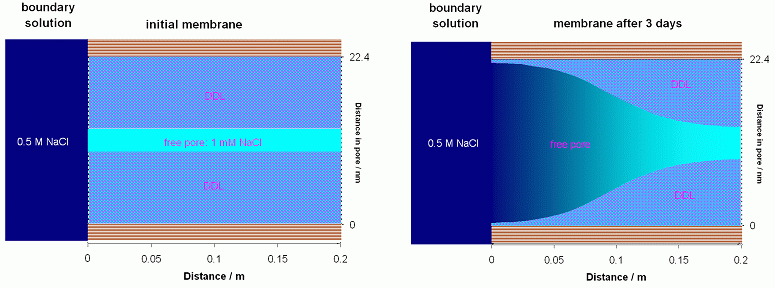
The figure shows how the extent of the DDL changes in 3 days time in
a 0.15 m thick clay liner, with the conditions noted in PHREEQC input file
membrane.phr.
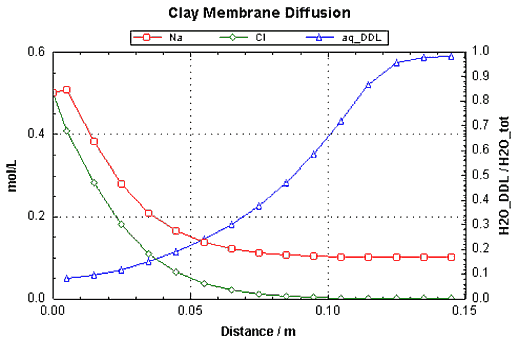
The graphic output from PHREEQC shows DDL water as a fraction of
total water on the secondary y_axis, and the total concentrations of Na
and Cl in the pores of the clay on the primary y_axis. The total concentrations are obtained
with the special BASIC statement sys("..."), which provides the total of moles in the solution and the donnan
layer on the surface. Note that total Na+ is more than total Cl- by the different amount in the donnan layer, where Na+ compensates the negative charge of 0.1 eq Su-.