
Dissolution of gibbsite proceeeds at low pH:
Al(OH)3 + 3 H+
↔ Al3+ + 3 H2O
And at high pH:
Al(OH)3 + OH-
↔ Al(OH)4-
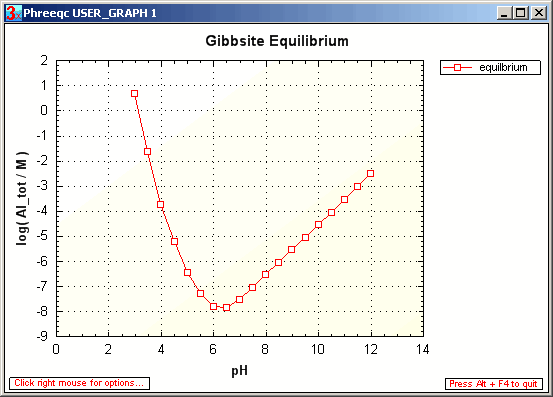
Accordingly, the total Al concentration in water is a function of the pH.
The PHREEQC input file gibbsite.phr calculates
the pH dependent Al concentration.
A number of waters are defined with increasing pH. The Al concentration is adapted to equilibrium with gibbsite.
The solutions are defined with USER_PUNCH in the file gibbsite.prn. This file is subsequently read in with
INCLUDE$ gibbsite.prn and processed.
The figure shows that the Al concentration may become quite high at low and high pH, in fact exceeding the drinking water limit of 0.2 mg Al / L.