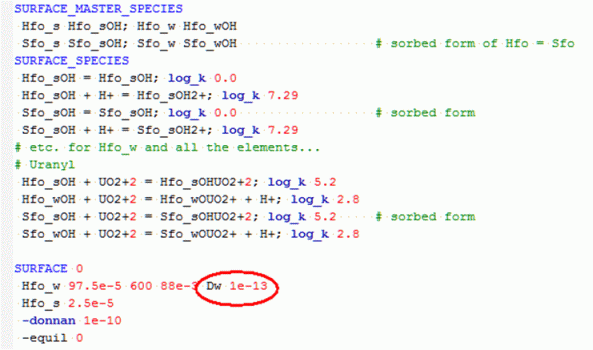

Strongly sorbing elements that are considered immobile, may be transported with colloids. The colloids consist of humic acids, oxy-hydroxide particles and small clay minerals, with sorbing properties that can be modeled with keyword SURFACE. New in version 2.13 is that SURFACEs can be transported by advective and dispersive transport (at the same pace as the other solutes) and by diffusion (with a diffusion coefficient that may differ from the other solutes, thus allowing for exclusion from zones inaccessible for the colloids).
How does it work?


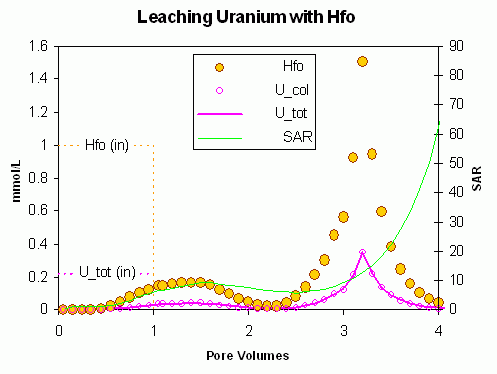
Transport of charged colloids is decisively influenced by the ratio of monovalent and divalent ions in solution. In fresh water, transport of colloids may become significant when the sodium adsorption ratio (SAR) increases above 11. We calculate a column example in which Uranium and ferrihydrite are injected in a Na/Ca solution with low SAR, followed by injection of a pure NaCl solution (PHREEQC input file colloid_U.phr). With low SAR, ferrihydrite is sorbed, and with it, also U disappears from solution since it is partitioned almost completely to the sorption sites of the colloid. When NaCl solution is injected, SAR increases, but initially the increase is buffered by Ca2+ from the diffuse double layer and the sorption sites of ferrihydrite. When SAR increases above the threshold value, all the sorbed ferrihydrite is released and the concentrations of ferrihydrite and U become quite high, higher than was injected originally with the influent.